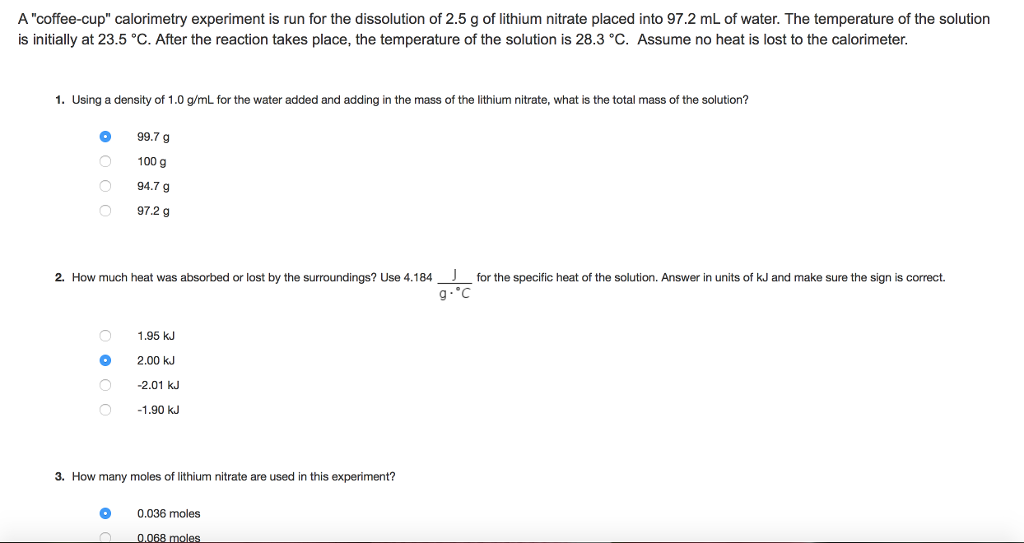
21+ Calculate The Mass Of The Reaction Mixture In The Calorimeter
Calorimetry is a science that pursues the theory and measurements of heat released or absorbed by a system caused by chemical reactions. 1 Heat given up by warm water.

How To Calculate The Mass Of Reaction In A Mixture Sciencing
Then calculate the mass of the solution based.

. To do this first determine the total volume of the solution based on the assumption that the volumes are additive and that. A calorimetric experiment uses essentially the same procedure except that the thermal energy change accompanying a chemical reaction is responsible for the change in. You can find the mass by measuring the volume of the solution and multiplying its volume by its.
Q 1000 g 188 C 4184 J g 1. A 626 g-reaction mixture that contains 015 moles of reactant A and causes a temperature increase of 46 C in a calorimeter has a heat of reaction of -80 kJmol. 1 calculate the mass of the contents in the calorimeter after the reaction for each trial.
Science Chemistry Calculate the mass of the reaction mixture in each reaction. Use 4184 J g 1 C 1 as the specific heat of water Solution. To do this first determine the total volume of the solution.
Calculate the heat capacity of the calorimeter in JC. Greater since taking the calorimeters heat capacity into account will compensate for the thermal energy transferred to the solution from the calorimeter. This approach includes the.
Calculate the mass of the reaction mixture in the calorimeter. The first ice calorimeter. For example when an exothermic reaction occurs in solution in a calorimeter the heat produced by the reaction is absorbed by the solution which increases its temperature.
The temperature of the reaction mixture is monitored as shown in the graph below. Basically you have a certain amount of water surrounding a reaction. D According to the graph.
Assume any loss of H2 gas is negligible if it did escape 1st Experiment Mass of Empty Calorimeter. You measure the temperature change in that water and you use that to calculate the heat gained or released. The solutions all originally at 200 C are combined in an insulated calorimeter.
Mass in calorimetry refers to the mass of the water or solution used in the experiment.

Calculate The Mass Of The Contents Of The Calorimeter G By Subtracting A From E Course Hero
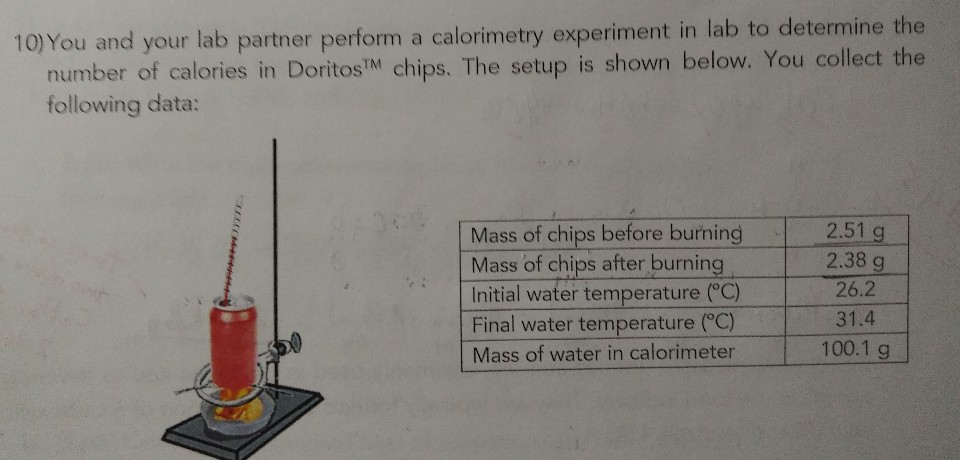
Answered 10 You And Your Lab Partner Perform A Bartleby

Solved Mass Of Calorimeter Cup I Nga Mass Of Water And Chegg Com
Browse Questions For Chemistry 101

How To Calculate The Heat Gained By The Calorimeter Sciencing

Solved Table 1 3 Mass Of Calorimeter Mass Of Calorimeter Chegg Com
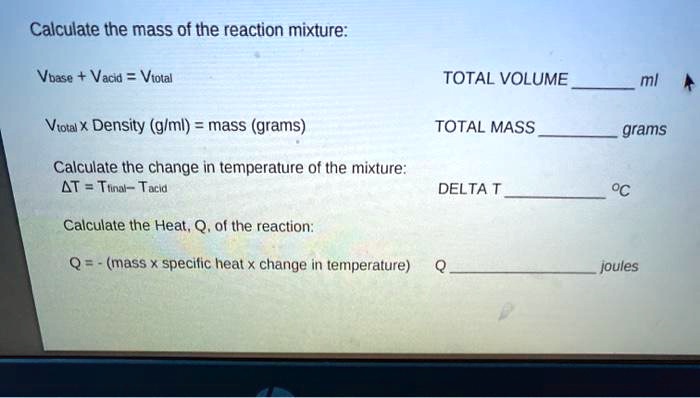
Solved Calculate The Mass Of The Reaction Mixture Vbase Vacid Violal Total Volume Mi Viotal Density Glml Mass Grams Total Mass Grams Calculate The Change In Temperature Of The
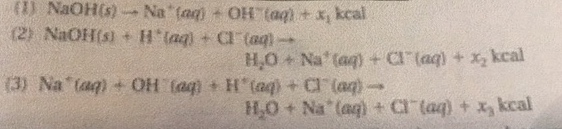
Solved Calculate The Mass Of The Reaction Mixture In Each Chegg Com

Experiment 6 Coffee Cup Calorimetry
Solved If The Lid Was Left Off During The Temperature Chegg Com

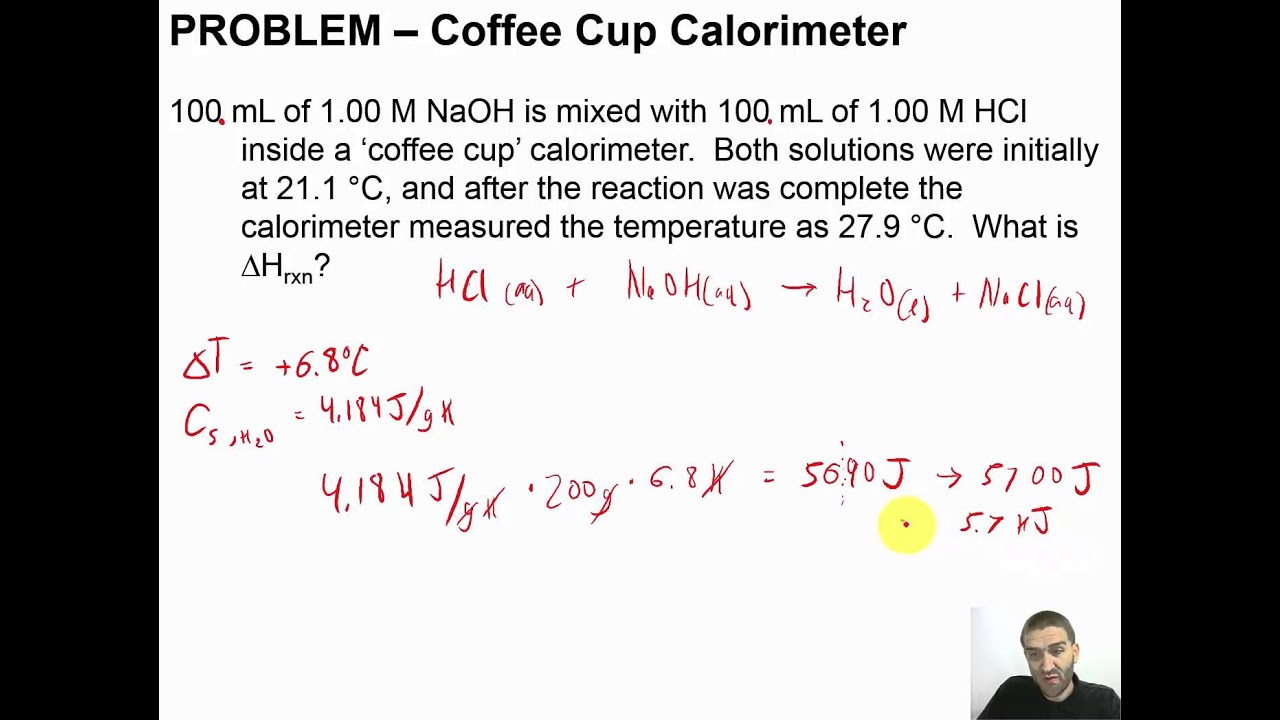
Solved Part A Exothermic Reaction Show All Calculation Setups Initial Temperature In Calorimeter Final Temperature In Calorimeter Volume Of 1 000 M Naoh Volume Of 1 000 M Hci Oml Sl Aml What Is The

Calorimetry Chemistry Socratic
Chapter 09 17 Problem Coffee Cup Calorimeter Youtube
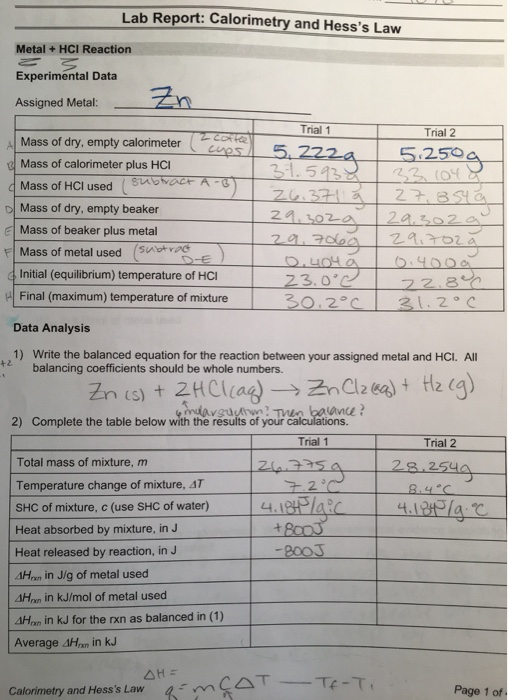
Solved Trying To Work Through My Lab Results But Not Sure Chegg Com

Calorimetry Chemistry Socratic
Rule Of Mixtures Calculator

Calculate The Mass Of The Contents Of The Calorimeter G By Subtracting A From E Course Hero